There continues to be strong demand for clinical and point-of-care diagnostics that determine markers for cancer, genetic diseases, pathogens, and for monitoring the efficacy of therapies. The availability of technologies for selective and sensitive determination of markers has become staggering, yet such technologies only superficially address what may be the most significant frontier in bioanalysis – the chemical dynamics within a living system, and even within a cell (for example, as associated with mRNA, and enzyme regulation for apoptosis). Enter the world of nanoparticle (NP) biosensors, where quantitative analysis can be achieved, multiplexed analysis is possible, and real-time reversible selective detection is done without separation and amplification. NPs represent one newer form of technology that is now being exploited in the labeling of tissues and within cells for the detection and tracking of biological markers, chemical signals and dynamic cellular events. We have been contributing to the area of learning to build functional NPs for bioassays. We are now in a position to launch the next step of technology evolution where the bioanalytical detection system concurrently becomes a platform for delivery of a cargo such as a therapeutic agent.
The role of NPs in bioanalytical technology in the next 5-10 years will be determined by the ability to create new benchmarks in assembly of functional materials that offer analytical sensitivity, selectivity, robustness, reusability, and simplicity, and that extend capability to beyond that of only contrast enhancement and passive sensing. We see a significant opportunity in the use of upconverting nanoparticles (UCNPs) as the basis of a NP technology that offers possibilities beyond those of quantum dots (QDs). UCNPs have already been touted as alternative NPs for a variety of bioanalytical applications, and these can also serve as contrast enhancement agents in MRI. Research targeted towards learning efficient, reproducible and practical methods of assembly of specific surface chemistries onto UCNPs to achieve functions related to optical bioassays and concurrent control of cargo delivery to influence environmental conditions is the primary interest described in this proposal.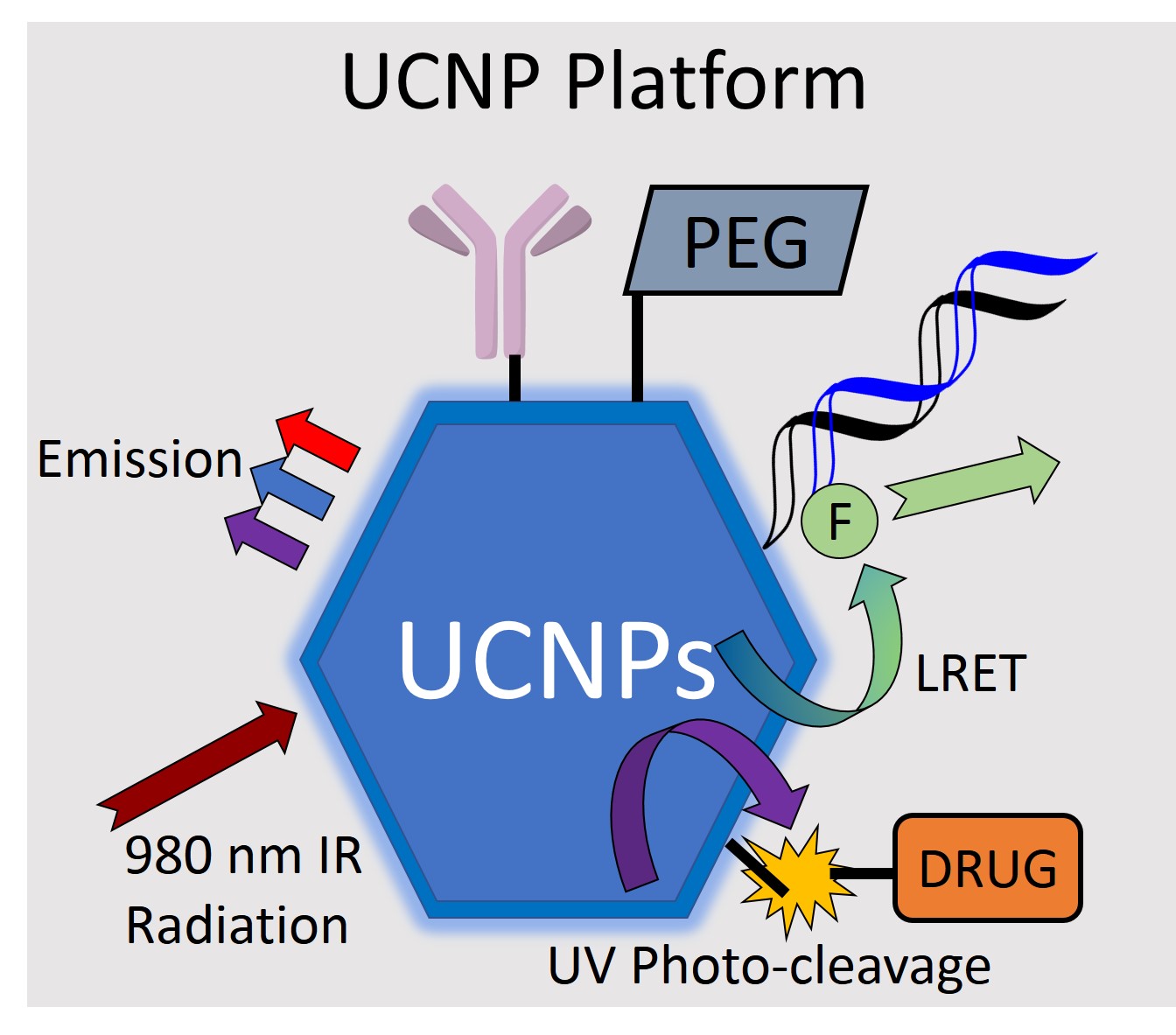
The lessons that have been learned from work done in Krull’s labs suggest that it is timely to re-evaluate limitations of bioprobes based on QDs. For example: QDs typically offer only a single strong emission band that still can have substantial width; surface instability of QDs cause time-dependent deterioration of fluorescence intensity; there are potential toxicity effects of heavy metals from QDs both in vivo and as contaminants introduced into the environment; and excitation energy is typically towards the UV-blue end of the optical spectrum. The operation of QD-bioprobes and SERS methods using optical excitation at shorter wavelengths can be problematic when considering biological samples, and environmental samples containing biologically derived components. Long wavelength excitation towards the infrared would be more suitable for penetrating into biological samples and reducing background, but offers insufficient energy for the desired optical scattering processes. A desire to move beyond passive sensing and actively trigger delivery of a cargo attached to a nanoparticle also is limited by insufficient energy at long wavelengths for stimulation of an optical mechanism of delivery. The need is for excitation at long wavelengths, while probing at a shorter wavelength for a bioassay, and excitation at even a shorter wavelength to induce photocleavage for cargo delivery.
An elegant approach to convert near-infrared (NIR) excitation radiation into UV or visible light can be achieved using upconversion nanoparticles (UCNPs). Core-shell UCNPs composed of NaYF4 nanocrystals (host matrix) doped with lanthanides such as Tm3+ (activator) and Yb3+ (sensitizer) convert continuous 980 nm laser light into a few sharp emissions of higher energy in UV/visible range. Upconversion is a process in which two or more photons are absorbed and combined through excited state absorption, energy transfer or photon avalanche. The upconversion luminescence originates from intra-configurational 4f transitions of lanthanide dopants and by varying the dopant concentration the emission spectra can be tuned. A primary advantage of UCNPs when considering multiphoton absorption is that energy levels are real and multiple photon absorptions occur sequentially rather than simultaneously, eliminating the necessity for high power density pulsed lasers. Recent studies have reported UCNPs as imaging agents for in vivo and ex vivo applications, and have noted photostability, minimal damage to living organisms, deeper light penetration into tissue, as well as an absence of background autofluorescence, thus improving sensitivity and signal-to-noise. UCNPs coated with targeting ligands were effective for in vivo imaging, without causing noticeable toxicity. Furthermore, imaging sensitivity of UCNPs in a side-by-side comparison with QDs (QD545, Invitrogen) demonstrated significantly superior performance. Similar to organic fluorophores and QDs, UCNPs can transfer energy to proximal gold nanoparticles, organic dyes and quantum dots via luminescence resonance energy transfer (LRET). This provides a transduction method analogous to that used in Krull’s FRET-based QD-bioprobes, retaining a high sensitivity to separation distance between a UCNP and acceptor fluorophore.
