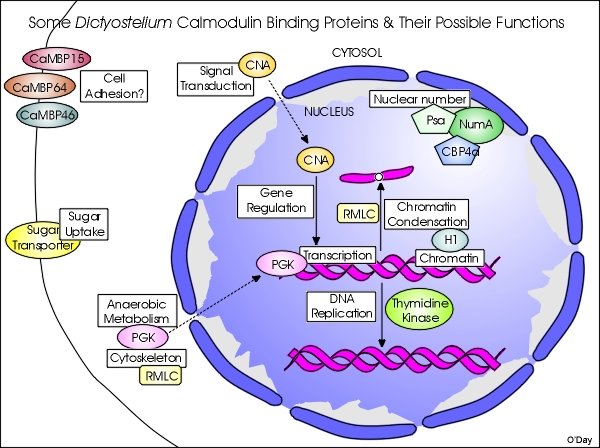
To date, only a handful of proteins have been verified as true CaMBPs in Dictyostelium and fewer CaM-binding domains have been experimentally determined. Some of the CaMBPs that we have isolated and begun to characterize are shown in the following graphic. While this is a work in progress, it outlines our attempts not only to characterize specific CaMBPs but define how their functions may be inter-related and co-regulated by calmodulin.
|
|
To date, nucleomorphin stands out as the best-characterized CaMBP in Dictyostelium and represents the first protein to have an experimentally identified NLS in this organism. The further characterization of the BRCT-domain protein nucleomorphin and its interactions with its binding proteins and co-regulation with other CaMBPs should provide further insight into nuclear functions during the cell cycle and other biomedically relevant processes. Other results also reveal that, in general, research on calmodulin and its binding proteins in Dictyostelium will have biomedical value. Since several of the CaMBPs of Dictyostelium are either highly similar to their mammalian counterparts and/or share CaM-binding domains that are highly conserved across species (e.g., thymidine kinase, phosphoglycerate kinase), studies of such proteins can provide valuable insight into their function in higher cells.
Last update: June, 2005. Unless otherwise stated, the information and graphics that are presented are the sole property of Danton H. O'Day, copyright 1998(c), 1999(c), 2000(c), 2001(c), 2002(c), 2003(c), 2004(c), 2005(c). If you would like permission to use any of the information contained in this website please contact Professor O'Day (doday@utm.utoronto.ca).
All information is the property of Danton H. O'Day, 2005© and may be used by individuals without prior permission if proper credit is given. If you would like to distribute or communicate this information to others in any form other than citing this website, you need to obtain permission.