Ongoing Research
Mercury contamination of Arctic foodwebs and ecosystems
Mercury (Hg) is a contaminant of global importance, impacting the health of wildlife and humans around the world. The most toxic and readily bioaccumulated form of Hg is methylmercury (MeHg), which is produced via the methylation of inorganic Hg(II) in aquatic ecosystems. My research has focused on identifying the sources of MeHg to Arctic freshwater and marine ecosystems. To this end, I have quantified, in various environmental compartments, the formation (methylation) and removal (demethylation) processes that ultimately dictate how much MeHg is available for bioaccumulation. To measure MeHg production, I have used various approaches ranging in scale from whole ecosystem MeHg mass-balances of wetland ponds on the tundra landscape, to microcosm (bottle and sediment core) experiments using novel enriched Hg stable isotope tracer techniques. Using enriched Hg stable isotopes to measure the kinetics of Hg transformations, I was able to determine that the methylation of inorganic Hg in Arctic marine waters is the primary source of methylated Hg species to local marine foodwebs and that long range transport of MeHg by ocean currents was unlikely to contribute significantly to the MeHg burden in coastal marine waters (Lehnherr et al., Nature Geoscience, 2011). I have also used stable isotope techniques to experimentally develop a mechanistic understanding of MeHg photodemethylation, which was applied to model this process at the whole-lake scale (Lehnherr & St. Louis, Environmental Science and Technology, 2009).
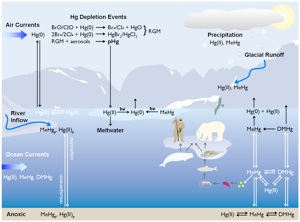
Being able to model integrated water column fluxes of MeHg photodemethylation was key in developing MeHg mass balance budgets for wetland ponds in the high Arctic. The results obtained from these mass-balances, which also incorporated atmospheric deposition, storage in the water column and bioaccumulation in zooplankton, showed that wetlands ponds on the Arctic landscape can be large producers of MeHg, similar in scale to boreal wetlands which are known to be significant sources of MeHg to downstream lake foodwebs (Lehnherr et al., Environmental Science & Technology, 2012a). Furthermore, methylation in these pond sediments is controlled by the availability of inorganic mercury (but surprisingly, not organic carbon or sulfate) and the activity of anaerobic bacteria such as methanogens, while MeHg concentrations in pond waters are controlled UV radiation through photodemethylation processes (Lehnherr et al., Environmental Science & Technology, 2012b).
| The Arctic Hg cycle. The complexity of transport and transformation processes occurring in the different compartments of Arctic ecosystems has hindered the elucidation of MeHg sources to local foodwebs |