Extracellular Calmodulin
The existence of extracellular calmodulin (eCaM) has had a long and controversial history. CaM is a ubiquitous calcium-binding protein that has been found in every eukaryotic cell system. Calcium-free apo-CaM and Ca2+/CaM exert their effects by binding to and regulating the activity of CaM-binding proteins (CaMBPs). Most of the research done to date on CaM and its CaMBPs has focused on their intracellular functions. The presence of extracellular CaM is well established in plants where it functions in proliferation, cell wall regeneration, gene regulation and germination. While CaM has been detected extracellularly in several animal species, including frog, rat, rabbit and human, its extracellular localization and functions are less well established. In contrast, the study of extracellular CaM in eukaryotic microbes remains to be done. We have shown that CaM is constitutively expressed and secreted throughout asexual development in Dictyostelium where the presence of extracellular CaM dose-dependently inhibits cell proliferation but increases cAMP mediated chemotaxis. During development, eCaM localizes within the slime sheath where it coexists with at least one extracellular CaMBP, the matricellular CaM-binding protein CyrA. Extracellular CaM also binds to and inhibits the proteolysis of extracellular CyrA further validating its function as a component of the ECM. Coupled with previous research, this work provides direct evidence for the existence of extracellular CaM in the Dictyostelium and provides insight into its functions in this model amoebozoan.
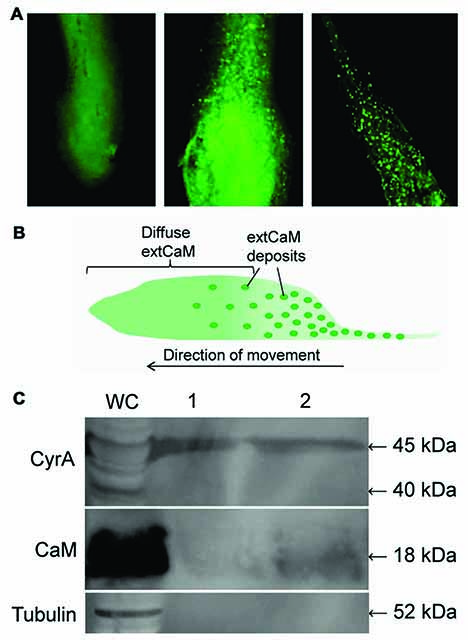
Figure. Detection of CyrA and CaM in the slime sheath. (A) Immunolocalization of DdCaM in AX3 pseudoplasmodium anterior (left panel), middle (center) and posterior sections (right). (B) Illustration of extCaM localization in pseudoplasmodium. (C) Presence of CyrA and CaM in isolated slime sheath deposited by WS380B slugs as shown via western blotting. Western blots probed with anti-C-CyrA, anti-CaM, or anti-tubulin.
Extracellular Matrix of Dictyostelium
The extracellular matrix (ECM) is a dynamic complex of glycoproteins, proteoglycans, carbohydrates, and collagen that serves as an interface between cells and their extracellular environment. Essential for normal cellular homeostasis, physiology, and development, it is also a key functionary in human diseases including cancer. The social amoebozoan Dictyostelium discoideum secretes an ECM during multicellular development that regulates aspects of multicellularity, cell motility, cell differentiation, and morphogenesis. It also provides structural support and protective layers to the resulting differentiated cell types. Proteolytic processing within the Dictyostelium ECM generates specific bioactive factors that regulate cell motility and differentiation. Dictyostelium ECM shares many features with mammalian and plant ECM, and thus presents an excellent system for studying the structure and function of the ECM. As a genetically tractable model organism, Dictyostelium offers the potential to further elucidate ECM functions, and to possibly reveal previously unknown roles for the ECM.

Figure. The ECM surrounding the multicellular slug and the cell types contained within. The sheath ECM, which is synthesized from the tip of the multicellular slug, is shed from the back of the slug as it migrates along the substratum. (Top panel) a diversity of processes occur in the ECM during slug migration. Cells secrete EcmA and EcmD which provide structure to the ECM. Secreted proteins such as AcbA and CyrA are processed into bioactive fragments that in turn bind to the cell surface to modulate cellular processes (e.g., spore differentiation and cell motility, respectively). (Bottom panel) different cell types within the slug sort to specific locations.
Calmodulin Binding Protein CyrA and Matricellular Dynamics

Figure. Summary of the known components and events mediated by the sheath ECM of Dictyostelium. Like mammals, during multicellular development, Dictyostelium cells release precursor proteins that are proteolytically processed to release bioactive peptides that regulate cell motility and differentiation. Matricellular proteins have been identified that regulate cell motility. Structural and cell adhesion proteins maintain the multicellular status of the aggregate, while the functional roles of the many signalling proteins remain to be analyzed. Based on research in mammals, matricellular proteins could function to mediate these signalling events. Other known roles of mammalian ECM proteins (e.g., morphogenetic functions) have so far not been sufficiently analyzed in Dictyostelium.
More about Robert Huber, Asst. Professor, Biology, Trent University:
http://huberlab.weebly.com/
References
- Huber, Robert and Danton H. O’Day, 2009. An EGF-like peptide sequence from Dictyostelium enhances cell motility and chemotaxis. Biochemical Biophysical Research Communications 379: 470-475
- Huber, Robert and Danton H. O’Day, 2011. EGF-like peptide-enhanced cell motility in Dictyostelium functions independently of the cAMP-mediated pathway and requires active Ca2+/calmodulin signalling. Cellular Signalling 23: 731-738
- Suarez, Andres, Robert Huber, Michael Myre, Danton H. O’Day, 2011. An extracellular matrix, calmodulin-binding protein with EGF-Like repeats that enhance cell motility. Cellular Signalling 23: 1197-1206.
- Ina Nikolaeva, Robert J. Huber and Danton H. O’Day, 2012. EGF-like peptide of Dictyostelium discoideum is not a chemoattractant but it does restore folate-mediated chemotaxis in the presence of signal transduction inhibitors. Peptides 34: 145-149
- Robert J. Huber and Danton H. O’Day, 2012. EGF-like peptide-enhanced cell movement in Dictyostelium is mediated by protein kinases and the activity of several cytoskeletal proteins. Cellular Signalling 24: 1770-1580. (doi:10.1016/j.cellsig.2012.05.004)
- Robert J. Huber, Andres Suarez, and Danton H. O’Day, 2012. CyrA, a matricellular protein that modulates cell motility in Dictyostelium discoideum. Matrix Biology 3: 271-280. (DOI: 10.1016/j.matbio.2012.02.003)
- Danton H. O’Day, Robert J. Huber and Andres Suarez, 2012. Extracellular Calmodulin regulates growth and cAMP-mediated chemotaxis in Dictyostelium discoideum. Biochemical Biophysical Research Communications 425: 750-754.
- Robert J. Huber and Danton H. O’Day, 2013. A Matricellular protein and EGF-like repeat signalling in the social amoebozoan Dictyostelium discoideum. Cellular and Molecular Life Sciences 69: 3989-3997 (DOI: 10.1007/s00018-012-1068-4)
- Danton H. O’Day and Robert J. Huber, 2013. Matricellular Signal Transduction Involving Calmodulin in the Social Amoebozoan Dictyostelium. Genes 4: 33-45
- Huber, R.J. and O’Day, D.H. (2015). Proteomic profiling of the extracellular matrix (slime sheath) of Dictyostelium discoideum. Proteomics 15: 3315-3319.
- Robert J. Huber, Danton H. O’Day, 2017. Extracellular matrix dynamics and functions in the social amoeba Dictyostelium: A critical review. Biochimica et Biophysica Acta 1861: 2971-2980.
- All information is the property of Danton H. O'Day, 2017© and may be used by individuals without prior permission if proper credit is given. If you would like to distribute or communicate this information to others in any form other than citing this website, you need to obtain permission.
Last Updated: