The one thing Dictyostelium discoideum can't do is form microcysts! On the other hand, most of the other species that have been studied can form these unicellular, dormant spheres. The microcysts of Dictyostelium mucoroides and Polysphondylium pallidum have been most actively studied. Currently, little work is being done on this cycle by any lab internationally.
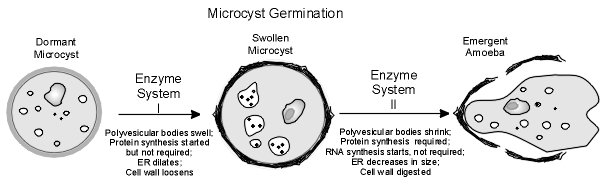
Microcyst formation can be induced by high osmotic pressure (e.g., 0.2M sucrose) or increasing concentrations of ammonia (Choi & O'Day, 1982. Develop. Biol. 92: 356-364). Such induction results in each individual cell rounding up and forming a cellulose-rich microcyst wall. The cells remain dormant as long as the stress is present. Simply washing the cells can lead to germination of the microcysts with each cyst releasing a single amoeba. Microcyst Germination involves a controlled sequence events beginning with swelling and followed by emergence.
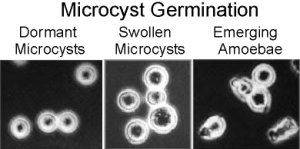 |
The time course of microcyst germination makes it especially appealing since the whole process from dormancy to emergence takes about 6 hours as seen in the following figure.
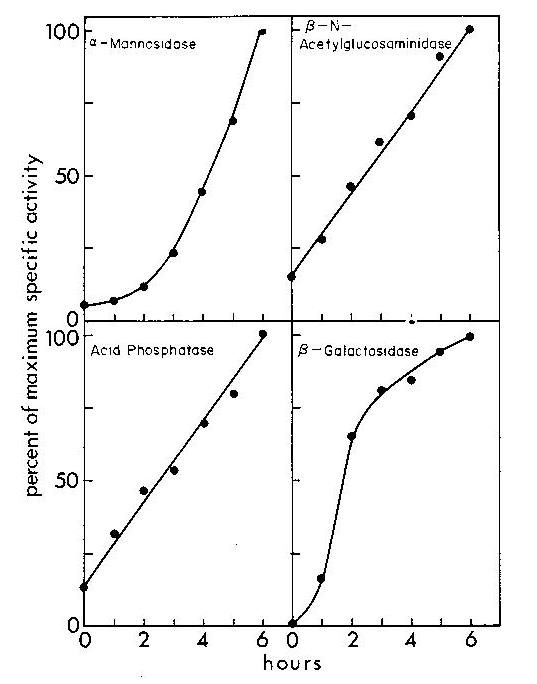
An extensive amount of work revealed the importance of lysosomal enzymes in modifying and removing the microcyst wall during microcyst formation and germination and that different populations of enzymes were under different regulatory controls (O'Day, 1973. J. Bacteriol. 113: 192-197; O'Day. and Francis, 1973. Can. J. Zool. 51: 301-310; O'Day, 1973. Exp. Cell Res. 79: 186-190; O'Day & Riley, 1973. Exp. Cell Res. 80: 245-249; O'Day, 1973. Cytobios 7: 223-232; O'Day, 1973. Develop. Biol. 36: 400-410; O'Day, D.H et al, 1976. Exp. Cell Res. 97: 358-364; O'Day, D.H., 1976. J. Bacteriol. 125: 8-13). For example, as shown in the following graphs, various enzymes accumulate in the extracellular medium during germination. Clearly these enzymes digest the cell wall as they are secreted from the cells and continue to digest it as they accumulate extracellularly.
This work led to the proposal of a model for microcyst germination that involves two successive enzyme systems as seen in the following figure.
|
|
Budniak, A. and O’Day, D.H., 2012. Microcysts: The Third Developmental Pathway of Social Amoebozoans. Protist 163: 2-14. (doi:10.1016/j.protis.2011.06.007)
Microcyst formation and germination, the least well studied of the three alternative developmental pathways open to the social amoebozoans, is also the simplest since it involves single cells. While classical studies profiled the morphological and biochemical changes that characterize microcyst differentiation, recent studies have focused on the molecular and evolutionary aspects of this system.
Figure 1. Amoebae of the cellular slime mould Polysphondylium pallidum may initiate one of three developmental pathways: fruiting body, macrocyst, or microcyst formation. Microcysts form individually while macrocysts and fruiting bodies are the results of multicellular development. Microcysts, macrocysts, and spores of fruiting bodies may germinate to release amoebae. Structures shown are not to-scale.
Microcyst differentiation (encystment) is primarily triggered by high osmolarity and to a lesser extent by starvation. The transformation of an amoeba into a microcyst involves the formation of a cellulose cell wall as well as specific enzymatic and biochemical changes leading to dormancy. The spherical microcyst remains dormant as long as high osmotic conditions persist.
Figure 2. High osmolarity triggers microcyst formation in Polysphondylium pallidum. The osmosensing adenylyl cyclase, ACG, is activated directly by high osmolyte concentrations (Saran and Schaap 2004). ACG produces cAMP, which activates protein kinase A (PKA) (van Es et al. 1996). PKA activity is sufficient for encystment (Ritchie et al. 2008), although its targets are unknown. This pathway also appears to maintain dormancy as long as high osmotic conditions persist (Ritchie et al. 2008).
Figure 3. Electron micrographs of encysting Polysphondylium pallidum cells during cyst wall formation. A.
The cell wall (CW) appears as fibrillar material outside the plasma membrane (large arrowhead). Numerous cellulose-rich secretory vesicles (SV) with a dense core (Co) in a less dense matrix (Ma) are observable near the cell periphery of young microcysts. B. Indentations (In) in the plasma membrane indicate that the vesicles fuse with it, releasing material during cell wall formation. Scale bar represents 1 micron.
When the osmolarity decreases, germination begins during which the cell wall is digested as the microcyst swells followed by the emergence of an amoeba. This rapid event typically takes 3-6 hours.
The relationship between encystment and sporulation, which has evolutionary significance, is also reviewed with particular emphasis on the role and regulation of actin tyrosine phosphorylation. In light of the recent sequencing of the genomes of microcyst-forming cellular slime mold species, which will generate more research on microcysts, this critical review covers past and recent research to set the stage for future work on this alternative developmental pathway.
References on Dictyostelium Microcysts
O'Day, D.H., 1973. a‑Mannosidase and microcyst differentiation in the cellular slime mould Polysphondylium pallidum. J. Bacteriol. 113: 192‑197.
O'Day, D.H. and D.W. Francis, 1973. Patterns of alkaline phosphatase activity during alternative developmental pathways in the cellular slime mould Polysphondylium pallidum. Can. J. Zool. 51: 301‑310.
O'Day, D.H., 1973. Intracellular and extracellular acetyl-glucosaminidase during microcyst differentiation in Polysphondylium pallidum Exp. Cell Res. 79: 186‑190.
O'Day, D.H. and L.J. Riley, 1973. Acid phosphatase activity during microcyst differentiation in the cellular slime mould Polysphondylium pallidum. Exp. Cell Res. 80: 245‑249.
O'Day, D.H. 1973. Intracellular and extracellular enzyme patterns during microcyst germination in the cellular slime mould Polysphondylium pallidum. Develop. Biol. 36: 400‑410.
Horgen, I.A., P.A. Horgen and D.H. O'Day, 1974. Purification and properties of acid phosphatase I from the cellular slime mould Polysphodylium pallidum. Can. J. Biochem. 52: 126‑136.
O'Day, D.H, D.I. Gwynne, and D.H. Blakey, 1976. Microcyst germination in the cellular slime mould, Polysphondylium pallidum: Effects of Actinomycin D and Cycloheximide on macromolecular synthesis and enzyme accumulation. Exp. Cell Res. 97: 358‑364.
O'Day, D.H., 1976. Acid protease during germination of the cellular slime mould, Polysphondylium pallidum. J. Bacteriol. 125: 8‑13.
Gwynne, D.I. and D.H. O'Day, 1978. RNA and protein synthetic patterns during germination of Polysphondylium pallidum microcysts. Can. J. Microbiol. 24: 480‑486.
O'Day, D.H. and G.D. Paterno, 1979. Intracellular and extracellular CM‑cellulase and b‑glucosidase during germination of Polysphondylium pallidum microcysts. Arch. Microbiol. 121: 231‑234.
Choi, A.H.C. and D.H. O'Day, 1982. Ammonia and the induction of microcyst differentiation in wild‑type and mutant strains of the cellular slime mould Polysphondylium pallidum. Develop. Biol. 92: 356‑364.
Choi, A.H.C. and D.H. O'Day, 1984. Calcofluor staining and cellulose formation during microcyst formation in Polysphondylium pallidum. J. Bact. 157: 291‑296.
All information is the property of Danton H. O'Day, 2017©, 2020© and may be used by individuals without prior permission if proper credit is given. If you would like to distribute or communicate this information to others in any form other than citing this website, you need to obtain permission.
Last update: January 3, 2020.