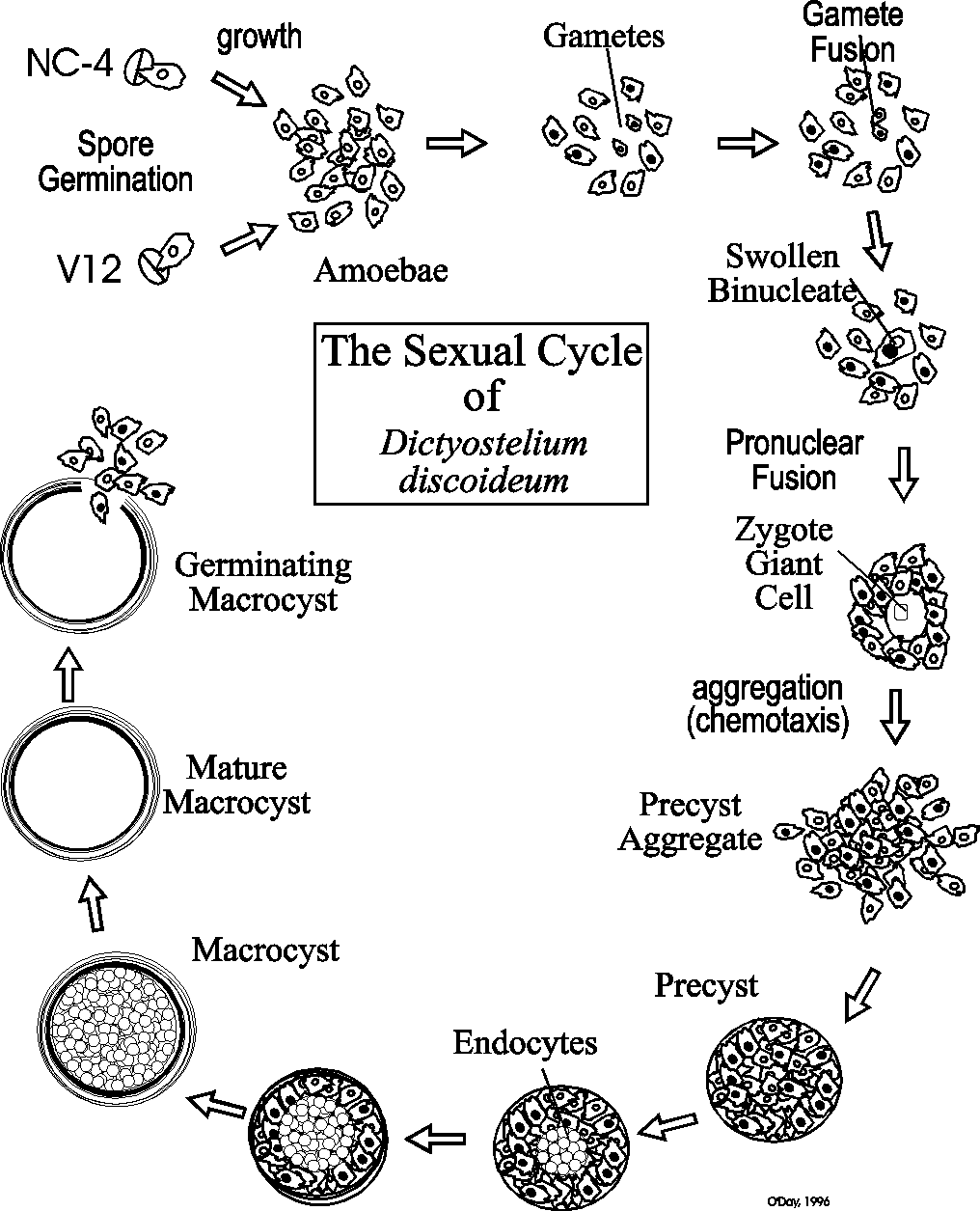
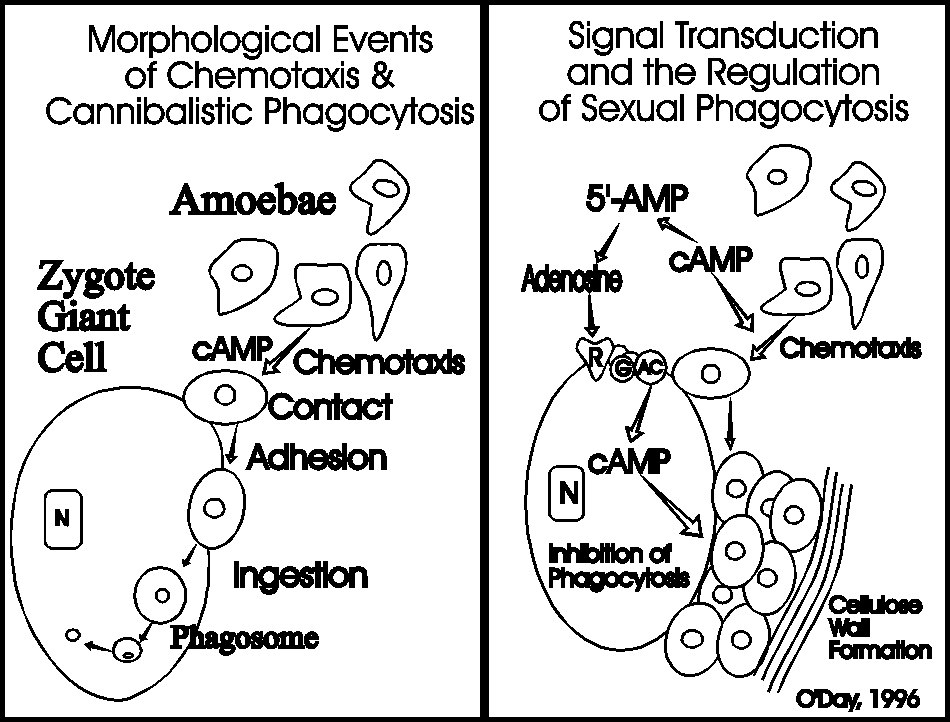
In 2012, O’Day and Keszei published a full review of sexual development in Biological Reviews that covered all of the research done by various groups. This was summarized in a figure that integrates all of the research to date.
O’Day, Danton & Alex Keszei, 2012. Signalling and Sex in the Social Amoebozoans. Biological Reviews 87: 313-329.
The sexual development of Dictyostelium has been comparatively ignored in spite of the many fundamentally important cellular and developmental events that occur during this alternative developmental mode. Sexual development occurs in the dark usually under wet conditions. Both homothallic and heterothallic mating types exist. But so far most research has been done on heterothallic mating between the two classic strains of Dictyostelium discoideum: NC4 and V12. As the following graphic reveals, when NC4 and V12 spores are cultured together in the dark, they undergo a phase of growth during which time small gametes appear. The gametes fuse ultimately forming a zygote giant cell that ingests surrounding cells to form the dormant macrocyst.
|
|
Let's now look at some of the details of each of these events:
Galloping Gametes
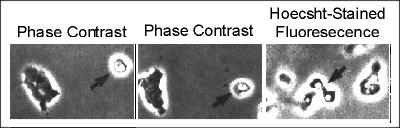
The gametes of Dictyostelium and other slime mould species are tiny cells with small nuclei that fluoresce brightly under UV light when stained with the DNA stain Hoescht 33258 as seen in the figure below. Evidence indicates that they are locked in G1 of the cell cycle which is typical of gametes of other species.
Movies of the cells show the gametes move comparatively quickly and when they come into contact protrusions form between them. The cells appear to fuse via these protrusions and once their cytoplasms coalesce their nuclei undergo a "sea urchin type" of pronuclear fusion (McConachie & O'Day, 1987. Can. J. Micro. 33: 1046-1049). Gamete formation does not require calcium ions but their fusion does. Comparative studies with several different species suggest that the gametes may be the source of sexual pheromone that initiates sexual development.
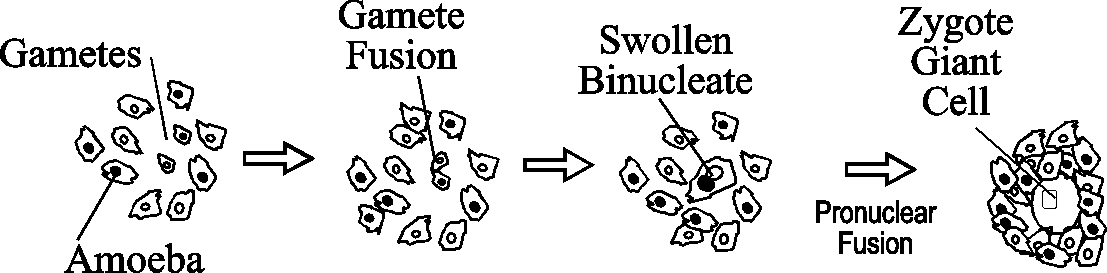
Shortly after their appearance, gametes fuse in pairs to form binucleates. Sometimes multinucleates form in a kind of "polyspermy". Within each binucleate the pronuclei swell, migrate together and fuse in a typical "sea urchin type" of fertilization. As this occurs, the cytoplasm of the binucleate is increasing in volume so that once the zygote nucleus forms, the cells are extremely large and are referred to as zygote giant cells.
The Zygote Giant Cells Take Control of Development
The zygote is designed for survival and becomes the controlling element for further development. First the giant cell secretes cyclic AMP to attract the non-zygotic amoebae to its surface.
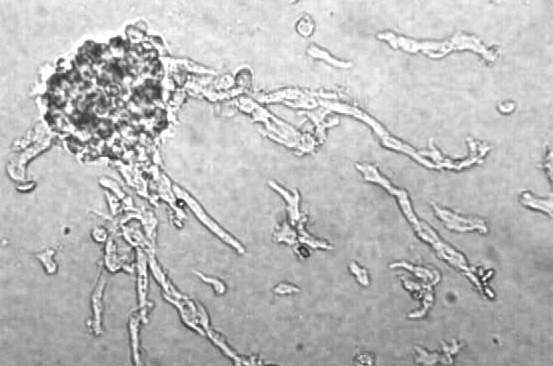
Figure Legend. Sexual aggregation in Dictyostelium is evident by the accumulation of amoebae around Zygote Giant Cells. The picture shows a naked Zygote contacting only a single amoeba. Five minutes later that same Zygote Giant Cell is surrounded by 6 cells and more have moved closer. If tracings of cells are made, it becomes clear that the amoebae show an oriented movement towards the zygote.
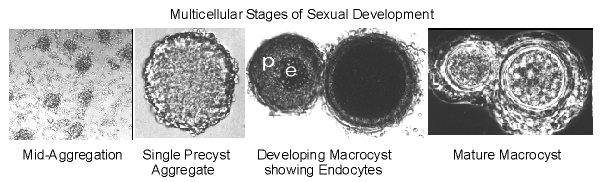
Upon their arrival the amoebae are ingested in an act of cannibalistic phagocytosis. The phagosomes containing the ingested amoebae are called endocytes. Chemotaxis occurs more rapidly than phagocytosis so the zygote giant cell becomes surrounded by hundreds of cells as a precyst aggregate. As the zygote continues to ingest the cells surrounding it, they are making their own prison by secreting a cellulose wall around the periphery of the precyst. Once the zygote has ingested all of the amoebae it secretes a denser more complex cyst wall before it enters a period of dormancy. Before germinating, the endocytes are digested, meiosis occurs and subsequent mitoses result in the production of many amoebae that emerge from the germinated cyst. The following photos show what some of these stages look like under the phase contrast microscope.
Volatile Pheromones Regulate Gamete Formation
Many years ago we discovered that Dictyostelium discoideum and many other cellular slime mould species produced sexual pheromones (O'Day and Lewis, 1975. Nature 254: 439; Lewis & O'Day, 1976. Can. J. Microbiol. 22: 1269-1273; Lewis & O'Day, 1977. Nature 268: 730-731; Lewis & O'Day, 1977. 1979. J. Bacteriol. 138: 251-253; O'Day, et al. 1987. Experientia 43: 619-621). The volatile nature of these pheromones kept us from biochemically analyzing them. However, the accumulated data suggest that the sexual pheromones regulate the formation of gametes.
cAMP Mediates Sexual Chemotaxis
Sexual chemotaxis is mediated by cyclic AMP. However, unlike asexual chemotaxis, there is comparatively little streaming during sexual development. This is likely due to low cell numbers because at high cell densities, pulsatile streaming that is reminiscent of asexual chemotaxis does occur. The following photo shows cells streaming towards a sexual precyst aggregate.
Cannibalistic Phagocytosis
Zygote Giant Cells attract and eat other amoebae. Like the tale of the sirens luring sailors to their death on the rocks, the zygote giant cells lure in unsuspecting amoebae only to dine upon them.
Figure Legend
Cannibalistic Sexual Phagocytosis in Dictyostelium is evident by the appearance of amoebae within vacuoles in the Zygote Giant Cell Cytoplasm. The picture shows a phagocytic Zygote Giant Cell under phase contrast microscopy revealing the presence of several amoeba in its cytoplasm. The proposed sequence of events leading to ingestion are shown (1-3). Inside the cytoplasm the "endocytes" or phagosomes undergo a continual decrease in size (4-6). After staining with Hoesch 33258 (photo on right) and visualization under fluorescence microscopy, the nuclei of the same Zygote Giant Cell is seen as a large bright region in the lower part of the cell. The amoebal nuclei decrease markedly in size once ingestion has occurred.
The key for the zygote is not to get to gluttonous because the incoming cells are not only going to be a source of nutrition, it is their job to lay down the first protective macrocyst layers. It turns out that the breakdown products of the chemoattractant, cyclic AMP, are designed to curb the zygotes appetite. As shown in the next figure, while cAMP serves as the chemoattractant to attract the zygotes food it also serves as the precursor to adenosine that inhibits the uptake of those same cells (Lewis et al, 1994. Cellular Signalling 6:209-215; Lewis & O'Day, 1994. Cellular Signalling 6:217-222). Secreted cAMP is sequentially broken down by phosphodiesterase to 5’AMP which in turn is dephosphorylated by a phosphatase to produce adenosine. The zygote giant cells possess adenosine receptors, which are A2-like purogenic receptors, that slow the rate of ingestion giving the attracted amoebae time to secrete a cellulose wall entraping themselves before they are eaten by the zygote giant cell.
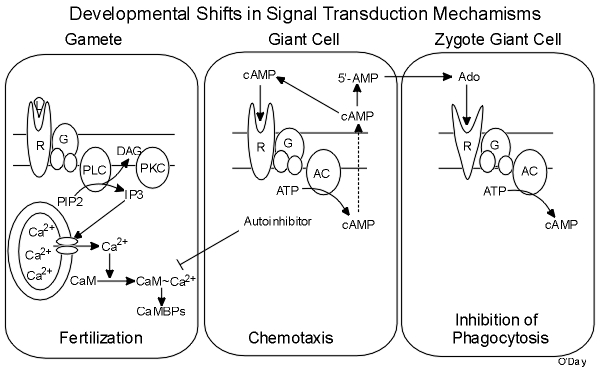
Developmental Shifts in Signal Transduction: Calcium & Calmodulin are Critical Regulators
Calcium plays a central role in regulating gamete fusion during sexual development (Szabo et al, 1982. Develop. Biol. 90: 375-382; McConachie & O'Day, 1986. Biochem. Cell Biol. 64: 1281-1287). In keeping with classic models, presumably the intracellular release of inositol trisphosphate (IP3) by receptor activation of G protein mediated phospholipase C (PLC) activity causes a release of calcium from intracellular stores. Being critical for cell fusion and pronuclear fusion, calcium operates by activating calmodulin (CaM) which then binds to and activated CaM-binding proteins (CaMBPs; Lydan & O'Day, 1993. Exp. Cell Res. 205:134-141). We use radiolabeled recombinant CaM to probe SDS-PAGE gels for CaMBPs in cell extracts (Lydan & O'Day, 1994. Chapter 36 In: Methods in Molecular Biology, Protocols for Gene Analysis 31: 389-396. J.M. Walker and A.J. Harwood, eds. Humana Press, Clifton N.J.). Coupled with patterns of CaM-dependent protein phosphorylation insight into important CaMBPs is emerging (Lydan & O'Day, 1993. Biochem. Biophys. Res. Commun. 192:1073-1078). CaM Kinase II (CaMKII) and Calcineurin (CN) are two such CaMBPs. Protein kinase C (PKC) is also important during fertilization and specific substrates have been revealed (Gunther et al, 1995. Exp. Cell Res. 220:325-331). A novel Galpha subunit has also been implicated in fertilization and phagocytosis (Browning & O'Day, 1995 Exp. Cell Research 219:709-716; Browning et al, 1993. Exp. Cell Res. 205:240-245).
More about Darren Browning, Professor, Department of Biochemistry & Molecular Biology, Medical College of Georgia: www.augusta.edu/mcg/bmb/browning-lab.php">http://www.augusta.edu/mcg/bmb/browning-lab.php">www.augusta.edu/mcg/bmb/browning-lab.php">http://www.augusta.edu/mcg/bmb/browning-lab.php
The Zygote Secretes an Autoinhibitor that Inhibits Calmodulin
Once the zygotes form they take over the control of subsequent development. They do this first by secreting a low molecular weight, hydrophobic autoinhibitor that feeds back to inhibit both gamete fusion and pronuclear fusion (O'Day, et al. 1981. Exp. Cell Res. 131: 456-458; Szabo & O'Day, 1984 Can. J. Biochem. Cell Biol. 62: 722-731; Lydan & O'Day, 1989. Biochem. Biophys. Res. Comm., 164: 1176-1181). Together these effectively prevent any new upstart zygotes from forming. The autoinhibitor has been shown to inhibit CaM so this feedback essentially terminates the early events of sexual development and initates the late events.
There is a Shift in Predominant Signalling Pathways
Using the information we have gleaned about signal transduction events during sexual developmental phases, we have been able to draw the following working model.
Thus a shift occurs in the predominant signal transduction pathways that mediate the successive phases of sexual development. These signaling pathways cross-talk to ensure the correct temporal flow of events. We are continuing to dissect these signaling pathways as we isolate the genes for new CaMBPs and knock them out to discover their cellular functions.
References on Dictystelium Sexual Development
O'Day, D.H., and K.E. Lewis, 1975. Diffusible mating‑type factors induce macrocyst development in Dictyostelium discoideum. Nature 254: 439.
Lewis, K.E. and D.H. O'Day, 1976. Sexual hormone in the cellular slime mould, Dictyostelium purpureum. Can. J. Microbiol. 22: 1269‑1273.
Lewis, K.E. and D.H. O'Day, 1977. The sex hormone of Dictyostelium discoideum is volatile. Nature 268: 730‑731.
O'Day, D.H. and A.J. Durston, 1979. Evidence for chemotaxis during mating in Dictyostelium discoideum. Can. J. Microbiol. 25: 542‑544.
Lewis, K.E. and D.H. O'Day, 1979. Evidence for a hierarchical mating system operating via pheromones in Dictyostelium giganteum. J. Bacteriol. 138: 251‑253.
O'Day, D.H., 1979. Aggregation during sexual development in Dictyostelium discoideum Can. J. Microbiol. 25: 1416‑1426.
Chagla, A.H., K.E. Lewis and D.H. O'Day, 1980. Ca++ and cell fusion during sexual development in liquid cultures of Dictyostelium discoideum. Exp. Cell Res. 126: 501‑505.
O'Day, D.H., S.P. Szabo, and A.H. Chagla. 1981. An auto‑inhibitor of zygote giant cell formation in Dictyostelium discoideum. Exp. Cell Res. 131: 456‑458.
Szabo, S.P., D.H. O'Day and A.H. Chagla, 1982. Cell fusion, nuclear fusion and giant cell differentiation during sexual development of Dictyostelium discoideum. Develop. Biol. 90: 375‑382.
Morris, S.P., K.E. Lewis and D.H. O'Day, 1982. Sexual pheromone production and response in agar‑plate and liquid cultures of Dictyostelium discoideum. Can. J. Microbiol. 28: 1273‑1276.
Szabo, S.P. and D.H. O'Day, 1983. The fusion of sexual nuclei. Biological Reviews 58: 323‑342.
Szabo, S.P. and D.H. O'Day, 1984. The low molecular weight autoinhibitor of sexual development in Dictyostelium discoideum inhibits both cell fusion and zygote differentiation. Can. J. Biochem. Cell Biol. 62: 722‑731.
Lewis, K.E. and D.H. O'Day, 1985. The regulation of sexual development in Dictyostelium discoideum: Cannibalistic Behaviour of the Giant Cell. Can. J. Microbiol. 31: 423‑435.
Lewis, K.E. and D.H. O'Day, 1986. Phagocytic specificity during sexual development in Dictyostelium discoideum. Can. J. Microbiol. 32: 79‑82.
McConachie, D.R. and D.H. O'Day, 1986. The immediate induction of extensive cell fusions by Ca++ addition in Dictyostelium discoideum. Biochem. Cell Biol. 64: 1281‑1287.
O'Day, D.H., J. Rivera and D.R. McConachie, 1987. Appearance and developmental kinetics of a unique cell type in Dictyostelium discoideum: Is it the gamete phase of sexual development? J. Exp. Zool. 242: 153‑159.
O'Day, D.H. and J. Rivera, 1987. Lectin binding and inhibition studies reveal the importance of D‑glucose, D‑Mannose and N‑Acetyl glucosamine during early sexual development of Dictyostelium discoideum. Cell Differentiation 20: 231‑237.
O'Day, D.H., R.R. Rama and M.A. Lydan 1987. Gamete formation reflects the sexual pheromone hierarchy of Dictyostelium giganteum. Experientia 43: 619‑621.
McConachie, D.R. and D.H. O'Day, 1987. Pronuclear migration, swelling and fusion during sexual development in Dictyostelium discoideum. Can. J. Micro. 33: 1046-1049.
Rivera, J. and D.H. O'Day, 1987. Chloroquine inhibits gamete fusion in Dictyostelium discoideum. Can. J. Microbiology. 33: 1125-1129.
Lydan, M.A., and D.H. O'Day, 1988. Developmental effects of the major ions found in a groundwater sample on sexual cultures of Dictyostelium discoideum. Can. J. Microbiol. 34: 207‑211.
Lydan, M.A., and D.H. O'Day, 1988. Intracellular calcium regulates cell fusion during early sexual development in Dictyostelium discoideum: Studies using A23187, CTC, La3+, IP3 and TMB‑8. J. Cell Sci. 90: 465-473.
Lydan, M.A., and D.H. O'Day, 1988. Different developmental functions for calmodulin in Dictyostelium discoideum: Trifluoperazine and R24571 both inhibit cell and pronuclear fusion but enhance gamete formation. Exp. Cell Res. 178: 51-63.
O'Day, D.H. and M.A. Lydan, 1989. The regulation of sexual development in Dictyostelium discoideum. Biochem Cell Biol. 67: 321-326. (invited)
Lydan, M.A. and D.H. O'Day, 1989. The autoinhibitor of cell and pronuclear fusion in Dictyostelium discoideum is a specific inhibitor of calmodulin. Biochem. Biophys. Res. Comm. 164: 1176-1181.
Browning, D.D. and D.H. O'Day, 1991. Con A and WGA binding glycoproteins associated with cell fusion and zygote differentiation in Dictyostelium discoideum: Effects of calcium ions and tunicamycin on glycoprotein profiles. Biochem. Cell Biol. 69: 202-290.
Browning, D.D., Lewis, K.L., and O'Day, D.H., 1992. Zygote giant cell differentiation in Dictyostelium discoideum: Biochemical Markers of Specific Stages of Sexual Development. Biochem Cell Biol. 70: 1200-1208 (invited).
Lydan, M.A. and D.H. O'Day, 1993. Calmodulin and calmodulin-binding proteins in Dictyostelium discoideum: Developmental regulation by calcium ions. Exp. Cell Res. 205: 134-141.
Lydan, M.A. and D.H. O'Day, 1993. Calmodulin-dependent protein phosphorylation and dephosphorylation during fertilization in Dictyostelium discoideum. Biochem. Biophys. Res. Commun. 192: 1073-1078.
Lewis, K.E., D.D. Browning and D.H. O'Day, 1994. Signal transduction during cannibalistic sexual phagocytosis: Calcium is not the trigger. Cellular Signalling 6: 209-215.
Lewis, Keith E. and Danton H. O'Day, 1994. Cannibalistic sexual phagocytosis in Dictyostelium discoideum is modulated by an A2-like receptor. Cellular Signalling Vol. 6: 217-222.
O'Day, D.H., K.E. Lewis and M.A. Lydan, 1995. Amoeboid gametes and fertilization in Dictyostelium: Gamete and pronuclear fusion are mediated by calmodulin and its binding proteins. In: Advances in Spermatozoal Taxonomy and Phylogeny. B.G.M. Jamieson, J. Ausio & J.-L. Justine, eds., pp. Memoires du Museum National d'Histoire Naturelle, Paris 166: 23-36.
Browning, D.D., and D.H. O'Day, 1995. G Protein Function during Biomembrane Fusion in Dictyostelium: Presence and Importance of a GaS Subunit during Fertilization and Phagocytosis. Exp. Cell Research 219: 709-716.
Gunther, K.E., S. Ramkissoon, M.A. Lydan, and D.H. O'Day, 1995. Fertilization in Dictyostelium: Pharmacological analyses and the Presence of a Substrate Protein Suggests Protein Kinase C is Essential for Gamete Fusion. Exp. Cell Res. 220: 325-331.
K.E. Lewis, and D.H. O'Day, 1996. Phagocytosis in Dictyostelium: Nibbling, Eating and Cannibalism. J. Eukaryotic Microbiology 43: 65-69. (invited).
Danton H. O’Day, 2017. Pheromones do regulate sexual development in Dictyostelium discoideum. J. Evol. Biol. 30: 2255-2256.
All information is the property of Danton H. O'Day, 2017© and may be used by individuals without prior permission if proper credit is given. If you would like to distribute or communicate this information to others in any form other than citing this website, you need to obtain permission.
Last update: August 2017; January 2020.