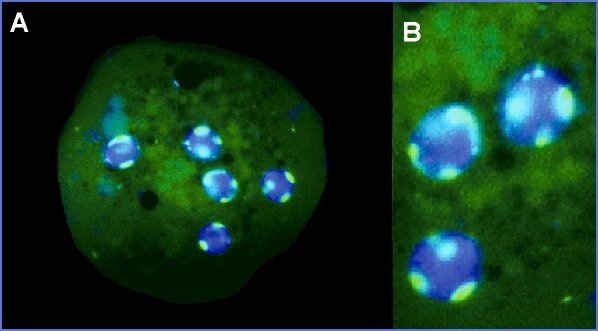
Nucleomorphin is a novel nuclear and nucleolar calmodulin (CaM) binding protein (CaMBP; Myre, Michael A. and Danton H. O'Day, 2002. Nucleomorphin: A novel, acidic, nuclear calmodulin-binding protein from Dictyostelium that regulates nuclear number. J. Biol. Chem. 277: 19735-19744). It is expressed as two main isoforms NumA1 and 2, which localize to the nucleoplasmic periphery as bright fluorescent patches as seen in the following photomicrographs. The nuclei are stained with Hoescht 332358 that fluoresces blue while the NumA is attached to green fluorescent protein (GFP) which, as its name implies, shows up green under fluorescence microscopy. It was subsequently revealed that these patches are the nucleoli of Dictyostelium.
GFP-Nucleomorphin (GFP-NumAΔDEED) localizes as patches within the nucleus.
Note: this form of nucleomorphin is lacking the DEED repeat thus generating multiple nuclei in cells.
Nucleomorphin is a multidomain protein. It has at least one bipartite NLS (48KKSYQDPEIIAHSRPRK66) and an experimentally verified single CaM-binding domain 171EDVSRFIKGKLLQKQQKIYKDLERF195) that interacts with CaM in both a Ca2+-dependent and Ca2+-independent manner. The NLS alone is responsible for directing nucleomorphin into the nucleus but how it localizes to the nuclear periphery as dense patches remains to be determined. The NLS alone can also localize GFP to the nucleus. This is the first demonstration of an NLS in Dictyostelium. NumA1 contains an extensive glu/asp or DEED repeat that regulates nuclear number. The removal of the DEED repeat results in cells with large numbers of nuclei. This multinuclearity is determined by nuclear activity and not by changes in cytoskeletal function (i.e., effects on cytokinesis).
Nucleomorphin: A multidomain protein
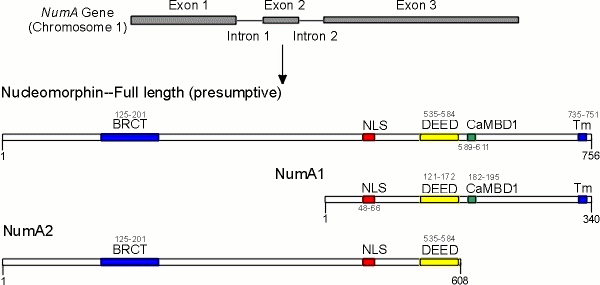
Yeast two hybrid and co-immunoprecipitation studies have identified calcium binding protein CBP4a as an interacting protein that binds to the DEED repeat. NumA2 also has a breast cancer C-terminus (BRCT) domain. Together the results are pointing to nucleomorphin as a critical regulator of nuclear number an event that is directly tied to nuclear events of the cell cycle. Details about each of these domains is contained in the following papers:; Myre, M. A., and D. H. O'Day, 2004b. Dictyostelium calcium-binding protein 4a interacts with Nucleomorphin, a BRCT-domain protein that regulates nuclear number. Biochemical Biophysical Research Communications 322: 665-671; Myre, M.A., and D.H. O'Day, 2004c. Dictyostelium nucleomorphin is a member of the BRCT-domain family of cell cycle checkpoint proteins. Biochimica Biophysica Acta, General Subjects.1675: 192-197; Myre, M.A., and D.H. O'Day, 2005. An N-terminal nuclear localization sequence but not the calmodulin-binding domain mediates nuclear localization of nucleomorphin, a protein that regulates nuclear number in Dictyostelium. Biochem. Biophys. Res. Commun. 332: 157-166).
NumA1 is the most well-studied nuclear/nucleolar protein in social amoebozoans and continued research has led to many interesting discoveries. Recent research has revealed more about NumA1-binding proteins and the translocation of these and other nuclear and nucleolar proteins and CaMBPs in Dictyostelium.
Last Updated: