Many Fundamental Biological Events Occur
Teachers can use Dictyostelium discoideum and other species to study many aspects of biology including chemotaxis and cell differentiation. The organism is available from many biological supply companies as well as from various researchers. The cells are easy and cheap to grow and maintain. For more information check out the Dictyostelium Web Site (http://dictybase.org) and the references cited therein. Also, see the Calmodulin Binding Proteins pages in this website for the latest information on these proteins in asexual development of Dictyostelium.
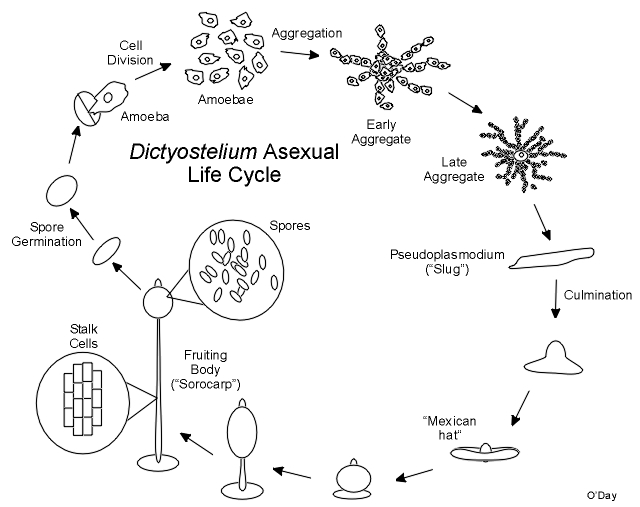
The Asexual Multicellular Fruiting Body Cycle
Growth occurs as cells feed on local bacteria. When the food supply is depleted, cells aggregate to form multicellular tissue-like aggregates as shown in the following graphic.
During this and all subsequent events the cells exist as individuals and do not fuse. As cells enter the aggregate it continues to grow upwards as a finger like projection. The tip of the aggregate is a strong source of cyclic AMP that plays a part in controlling mulitcellular development. Once complete the aggregate may fall over and migrate as a “slug” or pseudoplasmodium. The pseudoplasmodium (“pseudo” because unlike the plasmodia of “true” slime moulds which were discovered first, the cells remain separate and do not fuse) can orient towards light (phototaxis). Ultimately the front end of the pseuoplasmodium will stop as the back end catches up and culmination begins. Stalk cells form at the tip and flow through the cell mass in a reverse fountain pulling the remainder of the cells skyward. The stalk cells will die and serve to support the final spore mass at the top of the fruiting body (sorocarp). The spore mass consists of a drop of fluid within which are suspended viable spores that will start the next generation. In spite of what many biology texts say, there is no wall or case around the spore mass.
Two Types of Chemotaxis
Cellular slime moulds grow as solitary amoebae feeding on bacteria. The slime mould cells track down the bacteria by chemotaxis towards folic acid (see below) that is secreted by their prey. Once starved, the amoebae begin to aggregate together to form multicellular masses or aggregates. In Dictyostelium, this aggregation is a result of chemotaxis of amoebae to cAMP. Many labs are studying chemotaxis in Dictyostelium. Other species, such as Polysphondylium pallidum, are known to use other chemoattractants to mediate asexual chemotaxis. Comparative analyses in diverse species may shed some light on the essential components of this central biological process.
Chemotaxis in Polysphondylium pallidum
A Simple Bioassay for Chemotaxis
A quick and simple radial bioassay can be used to assess chemotactic competence to various chemoattractants during various stages of development. Drops of cells are simply place on the surface of a dilute agar containing the chemoattractant with appropriate controls. This method was derived based on early work by Bonner and Konijn (O'Day, 1979. Can. J. Microbiol. 25: 1416-1426). Recently this technique was used to show that protein tyrosine kinase activity is required for chemotaxis to folate but not cAMP (Browning et al, 1995. Cellular Signalling 7:481-489).
Different Species Add Insight to Complex Events
The cells remain separated in the multicellular aggregate as it continues to grow upward finally toppling over as a finger like slug or pseudoplasmodium. The slug can move and it can orient itself towards light (phototaxis). The multicellular aggregate and the slug have a prominent tip that is a source of cyclic AMP and is important in regulating the pattern of cell differentiation. When cells are cultured under water, no morphological tip forms on spherical aggregates. However, in Polysphondylium a biochemical tip forms which is the site at which stalk cell differentiation initiates (Paterno and O'Day, 1981. Can. J. Microbiol. 27: 924-936; Paterno and O'Day, 1982. Can. J. Microbiol. 28: 1143-1149). Furthermore, there is an overlap between the pre-stalk and prespore regions in pseudoplasmodia of Polysphondylium pallidum (O'Day, 1979. J. Cell Sci. 35: 203-215). Polysphodylium also forms small side-branches which in fact turn out to be mini-sorocarps which project perpendicularly from the main stalk giving this species the appearance of a spindly Christmas tree (O'Day & Durston, 1979. Can. J. Microbiol. 26: 959-964).
Making Spores to Start All Over
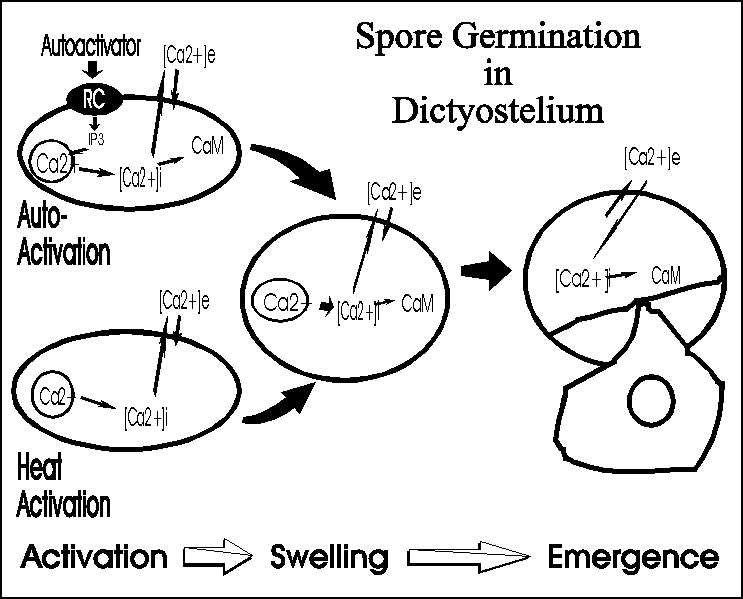
Once it stops moving, the slug changes shape as stalk cells begin to form at its tip, pushing through the cell mass to finally lift the cells off the substratum. In the end a lemon-shaped mass of spores resides at the top of a slender multicellular stalk. Under appropriate conditions the spores can germinate each releasing a single amoeba to renew the cycle. Calcium and calmodulin appear to be important regulators of dormancy and swelling during germination in Dictyostelium.
Actin tyrosine phosphorylation is regulated by the autoinhibitor during germination, likely because this cytoskeletal element plays some role during germination or is necessary once the amoebae emerge (Gauthier et al, 1997. Cellular Signalling 9:79-83).
Last update: June, 2005, January 3, 2020. Unless otherwise stated, the information and graphics that are presented are the sole property of Danton H. O'Day, copyright 1998(c), 1999(c), 2000(c), 2001(c), 2002(c), 2003(c), 2004(c), 2005(c), 2020(c). If you would like permission to use any of the information contained in this website please contact Professor O'Day (danton.oday@utoronto.ca).
All information is the property of Danton H. O'Day, 2005©, 2020(c) and may be used by individuals without prior permission if proper credit is given. If you would like to distribute or communicate this information to others in any form other than citing this website, you need to obtain permission.